Measurement of Uncertainty
While LIMS (Laboratory Information Management System) provides integrated data management that is important for pharmaceutical industry, evaluation of Measurement of Uncertainty is crucial to ensure the measured values are correct and do not get altered due to various operational conditions.
It is important to conduct Measurement of Uncertainty for Labs that is engaged in Pharma Industry to ensure the lifesaving products are produced to highest quality with Zero Defects.
Considering the importance for reliable measurement, ISO released ISO/IEC 17025 standard that talks about General requirements for the competence of Testing, & Calibration Laboratories that includes Evaluation of Measurement Uncertainty (Clause 7.6).
And ISO 21748 standard provides Guidance for the use of Repeatability, Reproducibility and Trueness estimates in measurement of uncertainty evaluation.
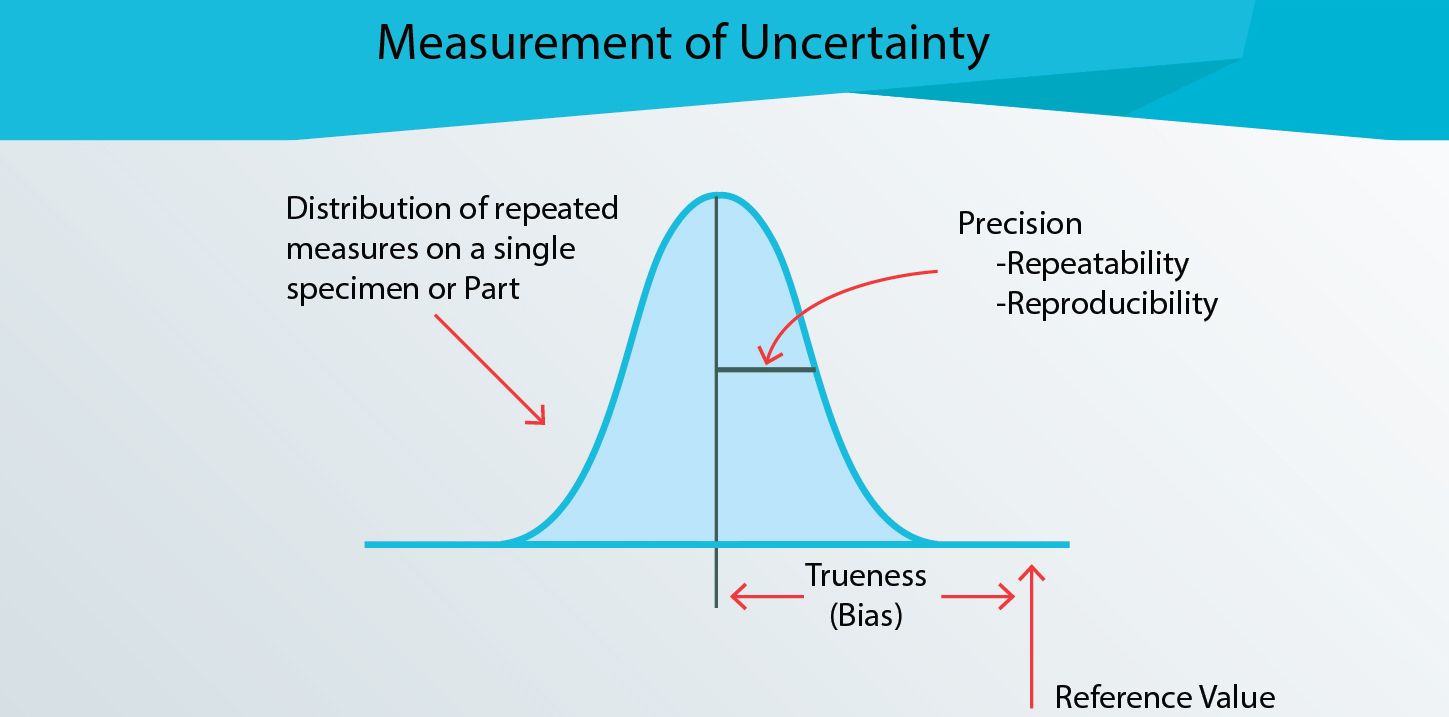
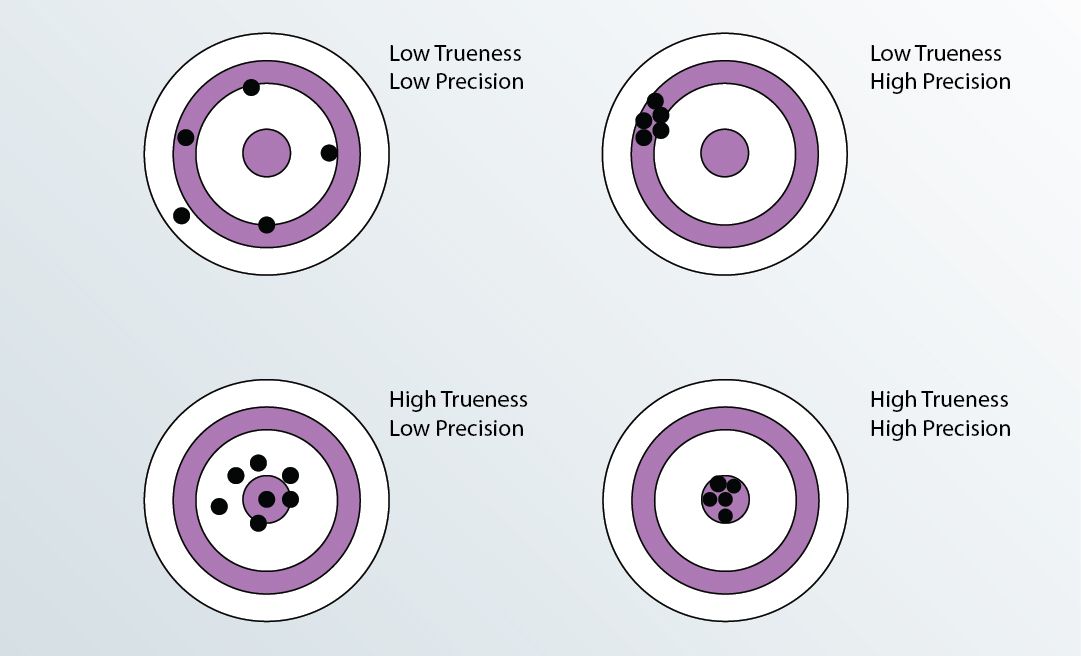
3 factors viz. Trueness, Repeatability and Reproducibility play major role in the evaluation of Measurement of Uncertainty. Let us understand how these 3 can affect the quality of results.
Trueness
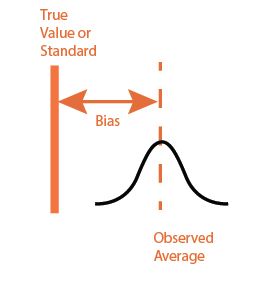
Trueness is the measurement of known standard reference sample and how close is the measured result. If the difference is beyond the allowable limits (published data), it is not advisable to use that instrument/equipment for testing or calibration. While the error can be addressed thru calibration, it is not recommended to use the ones with correction factors for calibration purpose.
Repeatability
Repeatability is the measurement under same test conditions that includes same operator, same instrument, same test conditions and studying the variations among the measured results.
Reproducibility
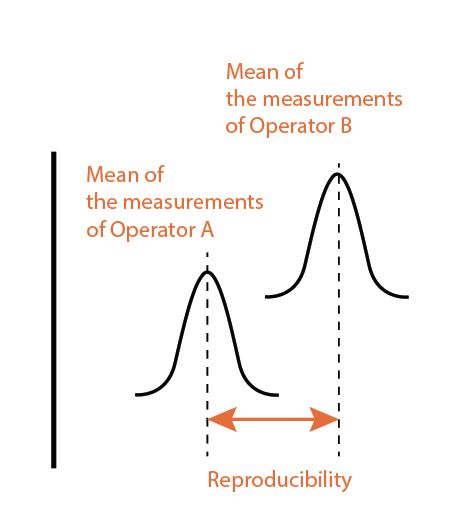
Reproducibility is the measurement using the same instrument but can be different operators and different location/test lab. Sometimes, the samples are measured in the process line instead of lab.
With high Repeatability, one can achieve high Reproducibility by ensuring robust test procedures & operators competencies.
Using the above data for an instrument/test equipment, the measurement of uncertainty can be arrived by comparing with published standards.
Once the uncertainty levels are arrived, one need to multiply with Coverage Factor (k) as per Industry requirement to report expanded measurement uncertainty for higher confidence of quality results. The Coverage Factors used for 95% confidence level is 2 and for 99% is 2.58.
CodonLIMS provides platform to capture the measurement of uncertainty data for each instrument / test equipment along with final uncertainty range. This data is made available when using instrument for any measurement and the results can be shown along with the uncertainty range.