Consumable Management System
What
This system provides options to track availability, expiration, usage and their respective work performance records in a secure and convenient package. With the help of this information, the management can reach the annual goal with low-cost efficiency and an effective outcome.
Why
By using the Consumable Management System, the management can track and prevent issues at the time of Research and development (R&D) or Manufacturing as mentioned below:
Availability and Expiration
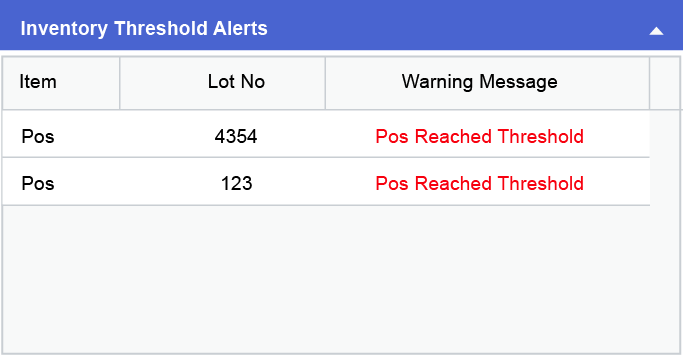
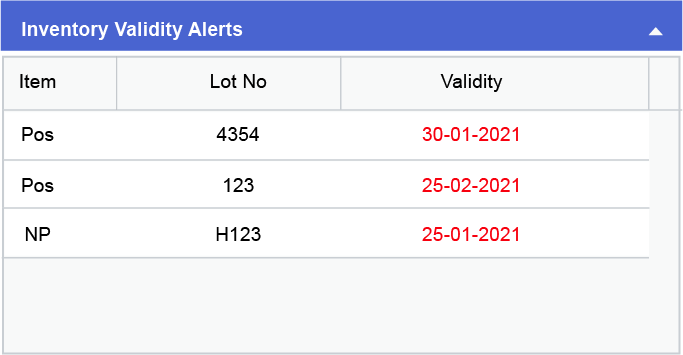
Inventory related issues such as shortage of samples, non-availability of certain inventory items or the items in stock having reached their date of expiry, could cause huge losses to the production house in terms of both money and time along with a delay in the product delivery. Through CodonLIMS the availability and expiration of items can be tracked by the user through dashboard and email alerts as shown in the images below:
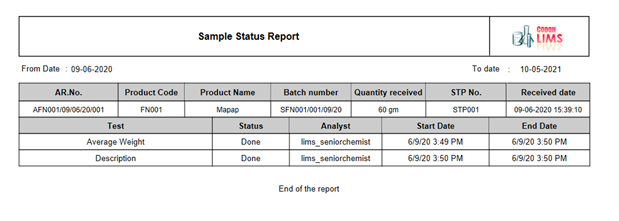
Usage
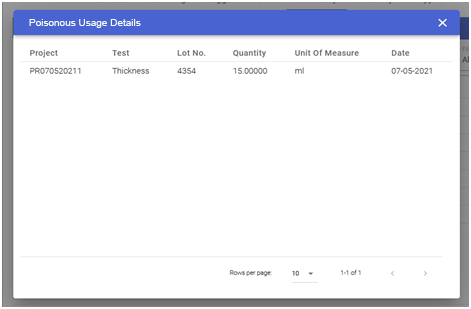
The management can track the inventory usage, making their availability visible. This information can help the management reduce wastage of their dead stock or overstock inventory which in turn will reduce the wastage of money. Usage tracking can also be done in the form of auditing. Through the usage tracking facility provided by the CodonLIMS application, the user can easily track the usage of inventory. They can find out or track the usage quantity and whereabouts of the inventory item at a given stage or test in production or R&D as shown in the image below:
Data Security
Authentication and authorization play a prominent role here. Only authorized users will be able to access the data related to inventory and consumables. They can also book or approve requests based on the role and permission accorded to the particular user. All important data like Purchase order (PO), validation reports and Certificate Of Analysis (COA) of suppliers can be stored safely and can be used for future reference.
Reference Records
Reference records are maintained for historical reasons. These records are approved by conducting tests or R&D of previous batches and should be stored in the system for reference in the future as required. Typical reference records include test results, Standard Operating Procedure (SOP), Standard Test Procedure (STP), COA reports or Analytical Test Data Sheet (ATDS) reports. These records help the user to validate the future lots or batches that get supplied to their organization.
CodonLIMS provides a platform that helps store and organize the data of inventory and alerting the management accordingly across different locations of the plant, based on the requests and approvals given by the users based on their roles.